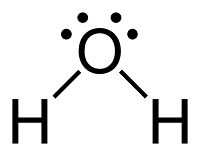
What is that picture? That is the lewis structure model of H2O, water. This diagram shows the bonding between atoms of a molecule and the pairs of electrons that exist within the molecule. This diagram is used to for showing covalently bonded molecules and compounds.
To draw this, you have to follow these steps:
Step 1: Count up the number of valence electrons for each atom, and total them up to give the total number of electrons for the molecule
Step 2: Determine which atom is the central atom and join all the atoms
using only single bonds. Sometimes which atom is the central atom is hard to determine
Step 3: Add lone pairs to each atom as necessary so that each atom has an octet (except H which can only have 2 electrons total) when you count all the atom's lone pairs and two electrons for each of its bonds.
Step 4: If the total you got in Step 1 is the same as in Step 3, you're done! If it doesn't you'll need to make some changes. If the number of electrons in Step 3 is larger than in Step 1, you must add double bonds as necessary between atoms. Then adjust the number of lone pairs again so that each atom has an octet. **Remember no double bonds to H or with any of the halogens!** If the total electron count with only single bonds is smaller than in Step 1, you probably made a mistake somewhere. Go back and double check.
Step 5: Continue adjusting the arrangement of single and double bonds and lone pairs (and also triple bonds if necessary) until the total electron count matches what you got in Step 1.