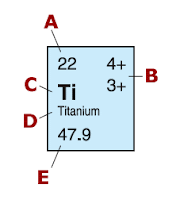
In Copper, its atomic number is 29. This means that there is 29 protons. The atomic mass is 63.5. You can make it 64. We then subtract 64 and 29. The result is 35 neutrons. From Titanium, its atomic number is 22. That means there are 22 protons. With a atomic mass of 48, we subtract the two numbers and get 26 neutrons.
What does the word isotope mean? An isotope is a variation of an atom with more or less neutrons. Isotopes are atoms that have lost/gain neutrons and also electrons. For example, Krypton has an atomic mass of 84. It can also be called Krypton-84. Sometimes you might see it written as Krypton-83 or Krypton-85. This means it lost and gained an electron. From Krypton-84 to Krypton-83 it lost a neutron. And from Krypton-84 to Krypton-85 it gained an electron. Sometimes you might see a number and a positive or negative symbol beside the atomic mass number. If there is no number or symbol beside the atomic mass number, this means the isotope is neutral. If you see a positive symbol and a number beside it, it means it has lost the number of electrons from the isotope making it positive by the number of charge because it lost that number of electrons. This means the isotope has more protons than electrons. And same goes for a negative number beside the atomic mass number. It means that the isotope gained the amount of electrons making the isotope negative because it has more electrons than protons. If there is just a negative or positive symbol, it means it has 1 less/more electron.
Examples:
How many electrons are in Ca2+?
For Ca, there is 20 protons. Since it is 2+, this means that the isotope is positively charged. The amount of electrons would be 18.
How many protons and electrons are in Cl-
Cl has 17 protons. This isotope is negatively charged. Remember that if it just has a negative sign, it means it lost 1 proton. That means there is 16 electrons in this isotope.
This is pretty much straight forward. No need to video... After doing the worksheets you should be fine with the stuff.



No comments:
Post a Comment