April. 20 Electron Configuration
HOLY Angelina JOLIE! Look at that diagram! That is how we write the electron configuration of any element. You write then how the arrows go down. So you start with 1s then 2s then 2p then 3s then 3p then 4s and so on. Remember this: the maximum amount of electrons in each shell are 2 for s, 6 for p, 10 for d, and 14 for f.
April 18 Atomic Mass and Isotopes
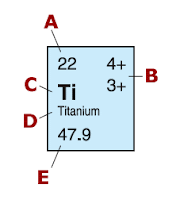
When you look at the periodic table of elements, you see that each element is in a box with numbers and a symbol. It goes from top to bottom as element name, atomic number, symbol, and atomic mass. The atomic number is the amount of protons that the element has and the atomic number is the mass of the atom. In order to get the mass, you add the amount of protons and neutrons. The reason why you add these two is because the nucleus of the atom contains only of protons and neutrons. You don't add electrons because they only orbit the atom. The protons are already given to you from the atomic number so all you need to do is subtract the atomic mass with the atomic number to get the number of neutrons. The atomic mass is usually shown as a decimal number in periodic tables because that the average mass of the atom. From the picture above, you can just say that Helium has an atomic mass of 4. That means it has 2 neutrons because it has 2 protons from the atomic number.
In Copper, its atomic number is 29. This means that there is 29 protons. The atomic mass is 63.5. You can make it 64. We then subtract 64 and 29. The result is 35 neutrons. From Titanium, its atomic number is 22. That means there are 22 protons. With a atomic mass of 48, we subtract the two numbers and get 26 neutrons.
What does the word isotope mean? An isotope is a variation of an atom with more or less neutrons. Isotopes are atoms that have lost/gain neutrons and also electrons. For example, Krypton has an atomic mass of 84. It can also be called Krypton-84. Sometimes you might see it written as Krypton-83 or Krypton-85. This means it lost and gained an electron. From Krypton-84 to Krypton-83 it lost a neutron. And from Krypton-84 to Krypton-85 it gained an electron. Sometimes you might see a number and a positive or negative symbol beside the atomic mass number. If there is no number or symbol beside the atomic mass number, this means the isotope is neutral. If you see a positive symbol and a number beside it, it means it has lost the number of electrons from the isotope making it positive by the number of charge because it lost that number of electrons. This means the isotope has more protons than electrons. And same goes for a negative number beside the atomic mass number. It means that the isotope gained the amount of electrons making the isotope negative because it has more electrons than protons. If there is just a negative or positive symbol, it means it has 1 less/more electron.
Examples:
How many electrons are in Ca2+?
For Ca, there is 20 protons. Since it is 2+, this means that the isotope is positively charged. The amount of electrons would be 18.
This is pretty much straight forward. No need to video... After doing the worksheets you should be fine with the stuff.
In Copper, its atomic number is 29. This means that there is 29 protons. The atomic mass is 63.5. You can make it 64. We then subtract 64 and 29. The result is 35 neutrons. From Titanium, its atomic number is 22. That means there are 22 protons. With a atomic mass of 48, we subtract the two numbers and get 26 neutrons.
What does the word isotope mean? An isotope is a variation of an atom with more or less neutrons. Isotopes are atoms that have lost/gain neutrons and also electrons. For example, Krypton has an atomic mass of 84. It can also be called Krypton-84. Sometimes you might see it written as Krypton-83 or Krypton-85. This means it lost and gained an electron. From Krypton-84 to Krypton-83 it lost a neutron. And from Krypton-84 to Krypton-85 it gained an electron. Sometimes you might see a number and a positive or negative symbol beside the atomic mass number. If there is no number or symbol beside the atomic mass number, this means the isotope is neutral. If you see a positive symbol and a number beside it, it means it has lost the number of electrons from the isotope making it positive by the number of charge because it lost that number of electrons. This means the isotope has more protons than electrons. And same goes for a negative number beside the atomic mass number. It means that the isotope gained the amount of electrons making the isotope negative because it has more electrons than protons. If there is just a negative or positive symbol, it means it has 1 less/more electron.
Examples:
How many electrons are in Ca2+?
For Ca, there is 20 protons. Since it is 2+, this means that the isotope is positively charged. The amount of electrons would be 18.
How many protons and electrons are in Cl-
Cl has 17 protons. This isotope is negatively charged. Remember that if it just has a negative sign, it means it lost 1 proton. That means there is 16 electrons in this isotope.
This is pretty much straight forward. No need to video... After doing the worksheets you should be fine with the stuff.
April. 14 The Atomic Theory
*LONG POST!!, IF DONT WANT TO READ, WATCH THE VIDEO AT THE BOTTOM OF THE PAGE :)*
How did we get from this:
To this:
The very first person to come up with the concept of the atomic theory was Democritus (460 BC - 370 BC). He came up with the thought that everything was made up of matter which was composed of atoms and they looked like tiny spheres. He thought it looked liked this:
Next Aristotle (384 BC - 322 BC) thought up that everything was made up of elements. They were fire, earth, air, water, and aether.
Antoine Lavoisier (Aug.26 1743 - May 8 1794) discovered that water is made of oxygen and hydrogen. He also discovered that chemical elements were neither destroyed or created, but just combined in different compounds in chemical reactions.
John Dalton (Sept. 6 1766 - July. 27 1844) proposed an atomic theory with five major important points. They were 1. Elements are made of tiny particles called atoms, 2. The atoms of a given elements are different from those of any other element, 3. All atoms of a given element are identical, 4. Atoms of one element can combine with atoms of other elements to form chemical compounds, 5. Atoms cannot be created, divided into smaller particles, nor destroyed in the chemical process.
Henri Becquerel (Dec. 15 1852 - Aug. 25 1908), Marie Curie ( Nov. 7 1867 - July 4 1934) and Pierre Currie (May 15 1859 - Apr. 19 1906) were three physicist who all three won the 1903 Nobel Prize in Physics for their discoveries of radioactivity. In 1911, Marie Currie won the 1911 Nobel Prize in Chemistry for her discovery of the elements Radium and Polonium.
J.J. Thompson (Dec. 18 1856 - Aug. 30 1940) was a physicist who is credited for the discovery of the electron and of isotopes and is the inventor of the mass spectrometer. In 1906, he won the Nobel Prize in Physics for the discovery of the electron and for his work on the conduction of electricity in gases.
Robert Millikan (Mar. 22 1868 - Dec. 19 1953) was an American physicist who discovered the charge on the electron which is negative. He measured the charge with his oil-drop experiment and confirmed that the charges were calculated to be 1.5924(17) x 10^-19.
Ernest Rutherford ( Aug. 30 1871 - Oct. 19 1937) was a New-Zealand born British chemist and physicist who became known as the father of nuclear physics. He discovered the concept of radioactive half life. He directed the Geiger-Marsden experiment in 1909 which proved J.J. Thompson's plum pudding model of the atom was incorrect. His model had the features of a high central charge concentrated in a very small volume in comparison to the rest of the atom and containing the bulk of the atomic mass which would be know as the atomic nucleus. He would also go on to discover the proton in 1919.
Niels Bohr (Oct. 7 1885 - Nov. 18 1962) was a Danish physicist who made contributions of understanding the atomic structure. He created the famous "Bohr Model" which shows the electrons and their orbits around the atom's nucleus.
Henry Moseley (Nov. 23 1887 - Aug. 10 1915) was an English physicist who contributed to concept of the atomic number. He also developed a law called Moseley's Law which also justified many concepts in chemistry by sorting the chemical elements of the Periodic Table of the Elements in a logical order based on physics.
James Chadwick (Oct. 20 1891 - July 24 1974) is known for his discovery of the neutron in 1932. His discovery of the neutron was crucial for the fission of uranium 235. He was later awarded the Nobel Prize in Physics in 1935 for his discovery of the neutron.
NOW FOR A VIDEO!! BONUS: SLIDESHOW OF THE ATOMIC THEORY WITH MILEY CYRUS'S PARTY IN THE USA... just instrumental though :( (Defiantly on the subject of Atomic Theory and Chemistry)TEHE
How did we get from this:
To this:
The very first person to come up with the concept of the atomic theory was Democritus (460 BC - 370 BC). He came up with the thought that everything was made up of matter which was composed of atoms and they looked like tiny spheres. He thought it looked liked this:
Next Aristotle (384 BC - 322 BC) thought up that everything was made up of elements. They were fire, earth, air, water, and aether.
John Dalton (Sept. 6 1766 - July. 27 1844) proposed an atomic theory with five major important points. They were 1. Elements are made of tiny particles called atoms, 2. The atoms of a given elements are different from those of any other element, 3. All atoms of a given element are identical, 4. Atoms of one element can combine with atoms of other elements to form chemical compounds, 5. Atoms cannot be created, divided into smaller particles, nor destroyed in the chemical process.
J.J. Thompson (Dec. 18 1856 - Aug. 30 1940) was a physicist who is credited for the discovery of the electron and of isotopes and is the inventor of the mass spectrometer. In 1906, he won the Nobel Prize in Physics for the discovery of the electron and for his work on the conduction of electricity in gases.
Niels Bohr (Oct. 7 1885 - Nov. 18 1962) was a Danish physicist who made contributions of understanding the atomic structure. He created the famous "Bohr Model" which shows the electrons and their orbits around the atom's nucleus.
Henry Moseley (Nov. 23 1887 - Aug. 10 1915) was an English physicist who contributed to concept of the atomic number. He also developed a law called Moseley's Law which also justified many concepts in chemistry by sorting the chemical elements of the Periodic Table of the Elements in a logical order based on physics.
James Chadwick (Oct. 20 1891 - July 24 1974) is known for his discovery of the neutron in 1932. His discovery of the neutron was crucial for the fission of uranium 235. He was later awarded the Nobel Prize in Physics in 1935 for his discovery of the neutron.
NOW FOR A VIDEO!! BONUS: SLIDESHOW OF THE ATOMIC THEORY WITH MILEY CYRUS'S PARTY IN THE USA... just instrumental though :( (Defiantly on the subject of Atomic Theory and Chemistry)TEHE
Subscribe to:
Comments (Atom)